Inventory Software for Medical Device Manufacturers
Inventory Software for Medical Device Manufacturers: Optimizing Supply Chain and Compliance
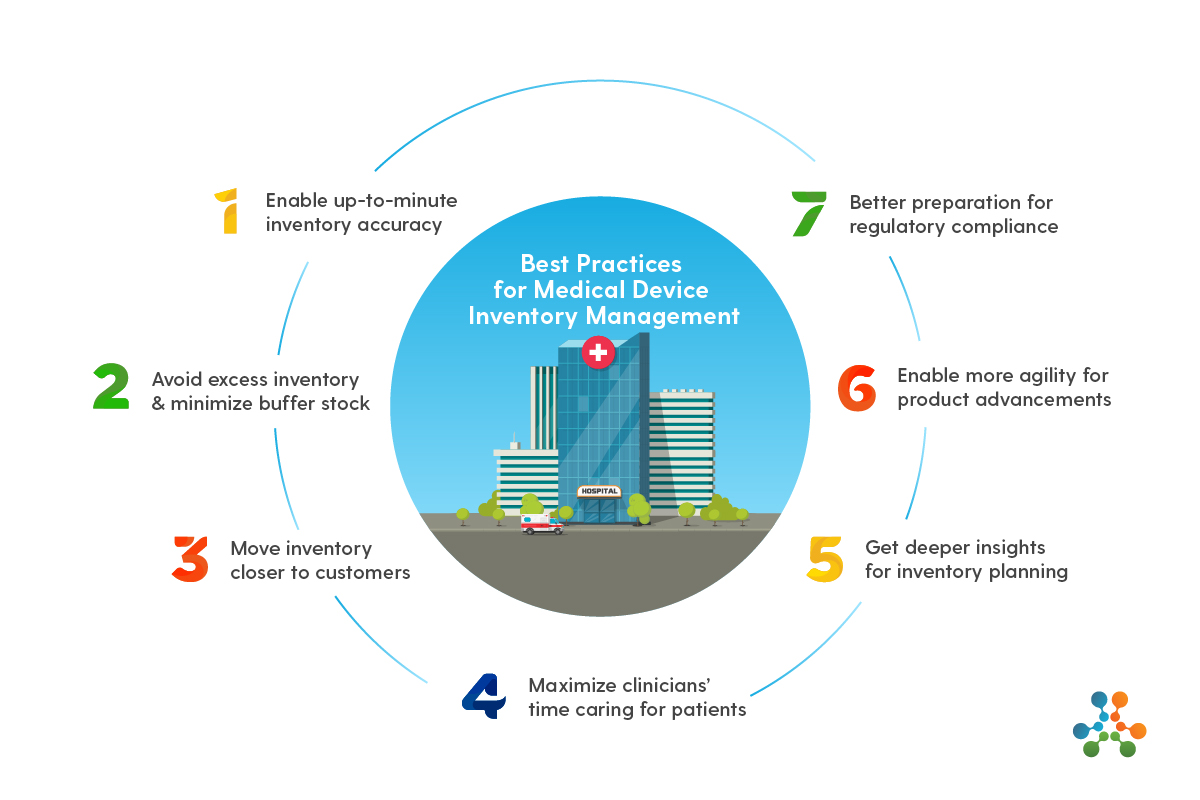
Medical device manufacturing is a highly regulated industry, and efficient inventory management is critical to ensure the timely production of devices, compliance with regulatory standards, and cost optimization. Medical device manufacturers must manage complex inventories that include raw materials, components, finished goods, and spare parts, all while adhering to stringent quality control measures. Inventory software specifically designed for medical device manufacturers addresses these challenges by automating processes, providing real-time visibility, and ensuring compliance with industry regulations.
In this article, we will explore the key features, benefits, and considerations for selecting the right inventory software for medical device manufacturers.
Key Features of Inventory Software for Medical Device Manufacturers
- Regulatory Compliance Management
- Inventory software for medical device manufacturers must help businesses comply with industry regulations, such as the FDA’s 21 CFR Part 820 and ISO 13485. This includes tracking device serial numbers, expiration dates, and lot numbers for each part used in production. The software ensures that all inventory transactions are recorded accurately, making it easier to maintain documentation for audits and inspections.
- Real-Time Inventory Tracking
- Medical device manufacturers deal with multiple parts, components, and finished products that need to be precisely tracked throughout the production process. Real-time inventory tracking features allow businesses to monitor stock levels, track materials across various production stages, and avoid delays caused by stockouts.
- Batch and Lot Tracking
- Batch and lot tracking is vital in the medical device industry to ensure traceability and recall management. Inventory software can track products by lot numbers or batch codes, ensuring that each component is traceable from production to distribution. This feature is especially important in case of product recalls or compliance audits.
- Expiration Date Management
- Many medical devices or components have expiration dates that must be carefully monitored. Inventory software for medical device manufacturers includes features to track expiration dates, ensuring that outdated inventory is used or discarded according to safety protocols. This minimizes waste and reduces the risk of using expired materials in the manufacturing process.
- Integrated Supply Chain Management
- Medical device manufacturers often work with multiple suppliers, so the ability to integrate inventory management with supply chain processes is essential. This includes features for order management, supplier lead time tracking, procurement automation, and vendor relationship management. Integration with suppliers ensures a smooth flow of materials, reducing the risk of stockouts and improving production schedules.
- Quality Control and Inspection Management
- Medical device manufacturers must adhere to strict quality control standards. Inventory software can integrate quality checks and inspections into the inventory process, ensuring that only approved materials and finished goods are used or shipped. This feature helps manufacturers maintain compliance with Good Manufacturing Practices (GMP) and other regulatory requirements.
- Multi-Warehouse and Multi-Location Support
- Many medical device manufacturers operate across multiple locations or warehouses. Inventory software with multi-warehouse support allows for centralized management of inventory, providing real-time visibility across all locations. This feature ensures that stock levels are consistent, orders are fulfilled on time, and excess stock is minimized.
- Demand Forecasting and Inventory Optimization
- Demand forecasting tools help medical device manufacturers predict future needs based on historical data, industry trends, and customer demand. This allows companies to maintain optimal inventory levels, ensuring that they have enough stock without over-purchasing, which can lead to storage costs and inefficiency.
- Barcode Scanning and RFID Integration
- The use of barcode scanning or RFID (Radio Frequency Identification) technology enhances inventory accuracy and efficiency. By scanning barcodes or RFID tags on components or products, manufacturers can easily track materials, reduce errors, and speed up processes such as receiving, stocking, and shipping.
- Integration with ERP Systems
- Medical device manufacturers often use Enterprise Resource Planning (ERP) systems to manage other business functions, such as accounting, sales, and procurement. Inventory software that integrates with ERP systems ensures seamless data flow across departments, providing accurate financial reporting and preventing discrepancies in inventory tracking.
Benefits of Inventory Software for Medical Device Manufacturers
- Improved Compliance
- Regulatory compliance is a major priority in the medical device industry. Inventory software helps manufacturers comply with global standards and regulations by maintaining detailed records of inventory movements, batch information, and product quality control. This makes it easier to undergo audits and ensure compliance with FDA, ISO, and other regulatory bodies.
- Enhanced Traceability
- Traceability is vital in the medical device industry to track the origins of each component used in the production process. Inventory software makes it easy to trace materials from suppliers through the manufacturing process, ensuring that each product is fully traceable for safety and recall purposes.
- Reduced Waste and Costs
- By efficiently tracking stock levels, expiration dates, and batch numbers, inventory software helps medical device manufacturers minimize waste caused by expired or obsolete components. The software helps optimize inventory management by reducing the risk of overstocking or stockouts, which can lead to unnecessary costs.
- Streamlined Operations
- With automated processes and real-time tracking, inventory software improves operational efficiency. By eliminating manual inventory checks, reducing human errors, and optimizing stock levels, medical device manufacturers can reduce lead times and speed up the production process.
- Increased Accuracy and Efficiency
- The ability to track inventory with barcode scanning, RFID technology, and automated data entry reduces manual errors, improves accuracy, and enhances overall operational efficiency. Manufacturers can avoid costly mistakes like incorrect shipments, production delays, and inventory discrepancies.
- Enhanced Customer Satisfaction
- By ensuring timely deliveries, accurate inventory levels, and traceability of products, inventory software improves customer satisfaction. Manufacturers can respond faster to customer demands, prevent stockouts, and meet delivery timelines, which leads to stronger customer relationships.
- Better Demand Planning
- Demand forecasting tools help medical device manufacturers predict future needs based on sales trends and historical data. This ensures that they can maintain optimal stock levels, avoid overstocking, and minimize excess inventory, resulting in cost savings and more efficient production cycles.
Considerations When Selecting Inventory Software for Medical Device Manufacturers
- Compliance Requirements
- Ensure that the inventory software complies with all relevant regulatory standards, such as FDA, ISO 13485, and Good Manufacturing Practices (GMP). The software should facilitate the documentation and traceability required for audits and inspections.
- Scalability
- As your business grows, your inventory management needs may change. Choose software that can scale to meet your future requirements, whether that involves managing additional warehouses, integrating with new suppliers, or handling higher product volumes.
- Customization Options
- Each medical device manufacturer has unique workflows and operational needs. Look for inventory software that can be customized to fit your specific processes, such as integrating with existing ERP systems or adapting to your quality control protocols.
- User-Friendliness
- Medical device manufacturers often have complex workflows, so choosing an inventory management system that is user-friendly and easy to navigate is essential. Training employees on how to use the system effectively is crucial for maximizing the software’s benefits.
- Support and Training
- Ensure that the software provider offers excellent customer support and training. The implementation of inventory software in a regulated industry like medical devices can be complex, so having reliable support is essential for troubleshooting issues and maintaining compliance.
- Integration with Existing Systems
- Many medical device manufacturers use ERP, accounting, and quality management systems. Make sure the inventory software can integrate with these systems to ensure a seamless flow of data across your operations.
Top Inventory Software for Medical Device Manufacturers
- SAP Business One
- Best For: Small to medium-sized medical device manufacturers.
- Features: Inventory control, batch and lot tracking, demand forecasting, integration with ERP, regulatory compliance management, and supply chain management.
- Pros: Scalable, highly customizable, and supports integration with various systems.
- Cons: Can be expensive for smaller businesses.
- Oracle NetSuite
- Best For: Larger medical device manufacturers with complex operations.
- Features: Real-time inventory tracking, automated procurement, regulatory compliance tools, quality control, and detailed reporting.
- Pros: Comprehensive and offers end-to-end solutions.
- Cons: Complex setup and requires training.
- Fishbowl Manufacturing
- Best For: Small to mid-sized manufacturers looking for an affordable solution.
- Features: Barcode scanning, inventory tracking, order management, batch control, and integration with QuickBooks.
- Pros: Affordable, easy to use, and integrates with accounting software.
- Cons: Limited scalability for large enterprises.
- FlexNet
- Best For: Medical device manufacturers focusing on regulatory compliance.
- Features: Batch and lot tracking, serial number tracking, inventory replenishment, and compliance with FDA regulations.
- Pros: Designed specifically for medical device manufacturers with an emphasis on compliance.
- Cons: Niche solution with limited features for non-medical device industries.
- Bluebird International
- Best For: Manufacturers looking for comprehensive and reliable supply chain management.
- Features: Inventory optimization, demand forecasting, serial number and lot tracking, regulatory compliance.
- Pros: Powerful features for managing complex inventory and supply chains.
- Cons: High initial cost and implementation time.
Conclusion
Inventory software for medical device manufacturers is an essential tool for streamlining operations, ensuring regulatory compliance, and optimizing inventory management processes. With features like batch tracking, expiration date management, and integration with supply chain systems, inventory software provides the visibility and control needed to maintain high standards of quality and efficiency in the production of medical devices. By selecting the right inventory management solution, manufacturers can reduce costs, improve operational efficiency, and ensure timely product delivery to meet customer needs.